Randomised Control Trials
INSIGHT trial
European (EU) Post Approval Study of the INCRAFT® AAA Stent Graft System in Subjects With Abdominal Aortic Aneurysms
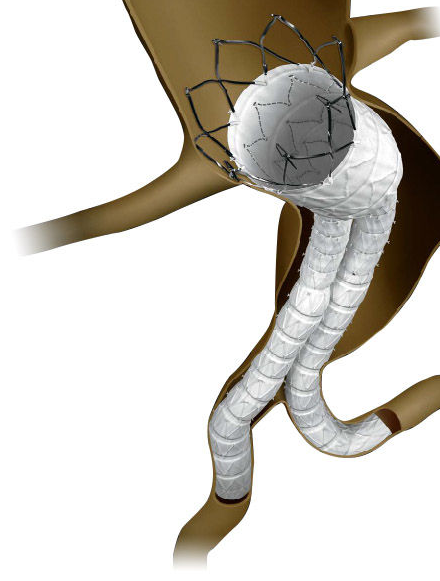
Cordis INCRAFT® AAA Stent Graft System
The Western Vascular Institute, along with 25 other European centres are involved in a post market surveillance, multi-centre EU clinical trial of the Cordis INCRAFT® abdominal aortic aneurysm (AAA) Stent-Graft System.
The aim of the trial is to evaluate the safety and effectiveness of INCRAFT device in patients with AAAs. Approximately 150 patients have been enrolled and will be followed over 5 years.
For more information click on clinicaltrials.gov
Clinical trial identifier: NCT02477111
MFMGR
About the MFM Global Registry
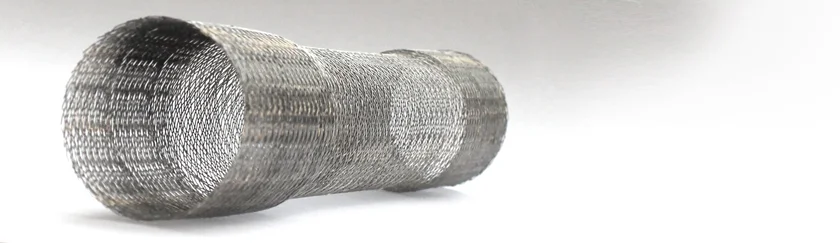
The Cardiatis Multilayer Flow Modulator is a disruptive technology. It is an innovative Stent which represents a new concept in the management of Aortic disease. The mode of action of this device is completely different from that of conventional aortic devices, which has caused much scepticism among the vascular community worldwide. However, positive clinical outcomes have forced a more thorough investigation of this new device and the team at the Western Vascular Institute have been commissioned to undertake this exploratory task.
The Western Vascular Institute is heading an independent registry, Multilayer Flow Modulator® Global Registry to collect data from the surgeons and interventionalists around the world who have used this stent to treat thoracoabdominal aortic pathologies. The experienced team at the Western Vascular Institute will objectively analyse the clinical outcomes in patients treated with this stent as well as engaging in independent biomedical testing of the device.
For more information click on MFMGR
Medtronic ENCHANT Trial
Endurant CHevAr New Indication Trial
The purpose of this clinical study is to further assess the clinical outcomes, safety, and performance of the Medtronic Endurant Chimney Graft Technique (Endurant Stent Graft Systems used with balloon-expandable covered stent grafts) for treatment of juxtarenal aortic aneurysms with a short infrarenal neck in a real-world setting. AAA can be treated by open surgery, a highly invasive procedure in which the diseased and bulged part of the abdominal aorta will be replaced by an artificial graft, thereby restoring the normal lumen of the aorta and excluding the blood pressure on the vessel wall of the bulging part of the aorta. Another form of treatment through which an abdominal aortic aneurysm can be treated is called endovascular aneurysm repair. An example of an endovascular device which is used for this treatment and which will also be used in this study is the Endurant II or Endurant IIs stent graft manufactured by Medtronic. The type of aneurysm which will be looked at in this study is called a juxtarenal aneurysm with a short infrarenal neck. One of the possible solutions for this is the Endurant Chimney Graft Technique. In this study, an Endurant II or IIs device will be placed inside the aorta to support the weakened part of the artery wall to release pressure from the bulge, thereby reducing the risk of the aneurysm rupturing. To avoid any blockage of the renal arteries when the stent graft is placed, one or two balloon-expandable covered stent grafts will also be inserted next to the Endurant II/IIs stent graft and inside part of one or both of the renal arteries. These stents going into the renal arteries will ensure that the blood flow to your kidneys remains intact.
For more information click on clinicaltrials.gov
Clinical trial identifier: NCT03320252
Images property of J&J, Medtronic and Cardiatis.